13943.The correct structure of the product A formed in the reaction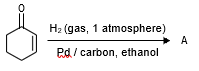
is
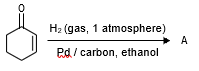
is
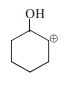
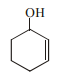
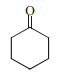
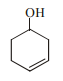
Explanation:


During hydrogenation of α, β unsaturated carbonyl compound by Pd catalyst selective reduction is observed of double bond.
13968.Which of the following pairs of ions is isoelectronic and isostructural?
ClO–
3 , SO2–
3
3 , SO2–
3
CO2–
3 , NO–
3
3 , NO–
3
ClO–
3 , CO2–
3
3 , CO2–
3
SO2–
3 , CO2–
3
3 , CO2–
3
Explanation:
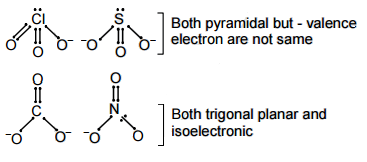
isoelectronic - same valence electron
isostructural - same structure

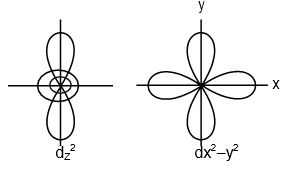
13971.Which of the following pairs of d-orbitals will have electron density along the axes?
dxy, dx2 - y2
dz2, dxz
dxz, dyz
dz2, dx2 - y2
Explanation:

13980.In pyrrole
the electron density is maximum on

the electron density is maximum on
2 and 5
2 and 3
3 and 4
2 and 4
Explanation:
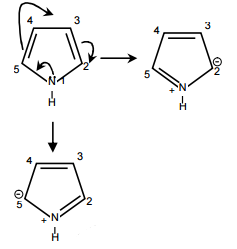

Electron density is maximum on 2nd & 5th carbon because –ve charge present at carbon 2 and 5 so electrophilic substitution reaction take place at 2nd and 5th carbon.
21204.The ratio of masses of oxygen and nitrogen in a particular gaseous mixture is 1 : 4. The ratio of number of their molecule is
7 : 32
1 : 8
3 : 16
1 : 4
21286.The electrons, identified by quantum numbers n and l
(i) n = 4, l = 1
(ii) n = 4, l = 0
(iii) n = 3, l = 2
(iv) n = 3, l = 1
can be placed in order of increasing energy, from the lowest to highest, as:
(i) n = 4, l = 1
(ii) n = 4, l = 0
(iii) n = 3, l = 2
(iv) n = 3, l = 1
can be placed in order of increasing energy, from the lowest to highest, as:
(iv) < (ii) < (iii) < (i)
(ii) < (iv) < (i) < (iii)
(i) < (iii) < (ii) < (iv)
(iii) < (i) < (iv) < (ii)
21305.The value of Planck’s constant is 6.63 × 10-34 J s. The velocity of light is 3.0 × 108 m s-1. Which value is closest to the wavelength in nanometers of a quantum of light with frequency of 8 × 1015 s-1?
5 × 10 s-18
4 × 10
3 × 107
2 × 10s-25
21315.β emission is always accompanied by
formation of antineutrino and α particle
emission of α particle and γ-ray
formation of antineutrino and γ-ray
formation of antineutrino and positron
21400.One mole of oxygen at 273 K and one mole of sulphur dioxide at 546 K are taken in two separate containers, then,
kinetic energy of O2 > kinetic energy of SO2
kinetic energy of O2 < kinetic energy of SO2
kinetic energy of both are equal
None of these
21423.The total pressure of a mixture of 6.4 grams of oxygen and 5.6 grams of nitrogen present in a 2 litre vessel is 1200 mm. What is the partial pressure (in mm) of nitrogen?
1200
600
900
200
21596.Regular use of which of the following fertilizers increases the acidity of the soil?
Ammonium sulphate
Superphosphate of lime
Urea
Potassium nitrate
21700.2-methylbutane on reacting with bromine in the presence of sunlight gives mainly
1–bromo 2–methylbutane
2–bromo 2–methylbutane
2–bromo 3–methylbutane
1–bromo 3–methylbutane
21763.In an antifluorite structure, cations occupy
tetrahedral voids
centre of cube
octahedral voids
corners of cube
21765.Which one of the following defects in the crystals lowers its density?
F–centres
Schottky defect
Frenkel defect
Interstitial defect
21799.A solution of two liquids boils at a temperature more than the boiling point of either of them. Hence, the binary solution shows ______.
negative deviation from Raoult's law
positive deviation from Raoult's law
no deviation from Raoult's law
positive or negative deviation from Raoult's law depending upon the composition
21819.An aqueous solution is 1.00 molal in KI. Which change will cause the vapour pressure of the solution to increase?
addition of 1.00 molal KI
addition of water
addition of NaCl
addition of Na2SO4
21843.A solution containing 10 g per dm3 of urea (molecular mass = 60) is isotonic with a 5% solution of a non–volatile solute. The molecular mass of this non–volatile solute is
300 g mol–1
350 g mol–1
200 g mol–1
250 g mol–1
21846.If α is the degree of dissociation of Na2SO4,the Van't Hoff factor (i) used for calculating the molecular mass is
1 – α
1 + α
1 – 2α
1 + 2α
21852.What is the volume (in ml) of 0.1 M potassium permanganate solution required tocompletely oxidize 100 ml of 0.5M ferrous sulphate solution in acidic medium?
20
200
50
100
22302.When KMnO4 acts as an oxidizing agent and ultimately forms MnO2–
4, MnO2, Mn2O3 and Mn2+, then the number of electrons transferred in each case is
4, MnO2, Mn2O3 and Mn2+, then the number of electrons transferred in each case is
1, 3, 4, 5
1, 5, 3, 7
4, 3, 1, 5
3, 5, 7, 1
22320.An infinite dilution of aqueous solution of BaCl2, molar conductivity of Ba2+ and Cl– ions are = 127.32 S cm2/moland 76.34 S cm2/mol respectively. What is Λ∞
m for BaCl2 at same dilute?
m for BaCl2 at same dilute?
280 S cm2mol–1
330.98 Scm2 mol–1
90.98 Scm2 mol–1
203.6 Scm2 mol–1
22332.When a lead storage battery is discharged
lead is formed
SO2is evolved
lead sulphate is consumed
sulphuric acid is consumed
22362.Consider an endothermic reaction, X → Y with activation energies Eb and Ef respectively for the backward and forward reactions, respectively. In general,
Eb < Ef
Eb > Ef
Eb = Ef
there is no definite relation between Eb and Ef
22380.The time for half life period of a certain reaction A → Products is 1 h. When the initial concentration of the reactant 'A' is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1, if it is a zero order reaction?
0.25 h
1 h
4 h
0.5 h
22389.The rate constants k1 and k2 for two different reactions are 1016 × e–2000/T and 1015 × e–1000/T, respectively. The temperature at which k1 = k2 is
$\dfrac{2000}{2.303}$ K
2000 K
$\dfrac{1000}{2.303}$ K
1000 K
22446.The colour of sky is due to
absorption of light by atmospheric gases
wavelength of scattered light
transmission of light
All of these
22482.Gold is extracted by hydro metallurgical process, based on its property
of being electropositive
of being less reactive
to form complexes which ani water soluble
to form salts which are water soluble
22526.In which of the following ionization processes the bond energy increases and the magnetic behaviour changes from paramagnetic to diamagnetic?
N2 → N2+
O2 → O2+
C2 → C2+
NO → NO+
22533.For the given complex [CoCl2(en)(NH3)2]+, the number of geometrical isomers, the number of optical isomers and total number of isomers of all type possible respectively are
2, 2 and 4
2, 2 and 3
2, 0 and 2
0, 2 and 2
22536.The IUPAC name of K2[Ni(CN)4]is ______.
Potassium tetracyanatonickelate (II)
Potassium tetracyanonickelate (II)
Potassium tetracyanonickel (III)
Potassium tetracyanatonickel (II)
22573.Excess of silver nitrate solution is added to 100 ml of 0.01 M Pentaaqua chloro chromium(III) chloride solution. The mass of silver chloride obtained in grams is [Atomic mass of silver is 108].
287 × 10–3
143.5 × 10–3
143.5 × 10–2
287 × 10–2
22623.Methyl phenyl ether can be obtained by reacting
phenolate ions and methyl iodide
methoxide ions and bromobenzene
methanol and phenol
bromobenzene and methyl bromide
22647.Which of the following compounds when heated with CO at 150°C and 500 atm pressure in presence of BF3 forms ethyl propionate?
C2H5OH
CH3OCH3
C2H5OC2H5
CH3OC2H5
22864.Bakelite is a polymer of
benzaldehyde and phenol
formaldehyde and phenol
formaldehyde and benzyl alcohol
acetaldehyde and phenol
22900.Which one of the following statements about aspirin is not true?
It belongs to narcotic analgesics.
It is effective in relieving pain.
It has anti blood clotting action.
It is a neurologically active drug.
