22670.CH3CHO and C6H5CH2CHO can be distinguished chemically by:
Tollen's reagent test
Fehling solution test
Benedict test
Iodoform test
22671.Which of the following acids does not exhibit optical isomerism?
Lactic acid
Tartaric acid
Maleic acid
α–amino acids
22672.A mixture of benzaldehyde and formaldehyde on heating with aqueous NaOH solution gives
benzyl alcohol and sodium formate
sodium benzoate and methyl alcohol
sodium benzoate and sodium formate
benzyl alcohol and methyl alcohol
22674.Which of the following reactions will not result in the formation of carbon–carbon bond?
Reimer–Tieman reaction
Friedel Crafts acylation
Wurtz reaction
Cannizzaro reaction
22675.The correct sequence of steps involved in the mechanism of Cannizzaro's reaction is _____.
nucleophilic attack, transfer of H– and transfer of H+
electrophilic attack by OH–, transfer of H+ and transfer of H–
transfer of H–, transfer of H+ and nucleophilic attack
transfer of H+, nucleophilic attack and transfer of H–
22676.Identify the product in the reaction
$PhC\equiv CMe\;\;{\xrightarrow{\;H_3O^+,\; Hg^{2+}\;}}\;\;?$
PhCH2CH2CHO
PhCOCH2CH3
PhCH2COCH3
PhCOCOMe
22678.An organic compound X is oxidized by using acidified K2Cr2O7. The product obtained reacts with phenyl hydrazine but does not answer silver mirror test. The possible structure of X is
(CH3)2CHOH
CH3CHO
CH3CH2OH
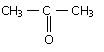
22679.In the following sequence of reactions, the alkene affords the compound 'B'
CH3CH=CHCH3 $\xrightarrow{O_3} \text{A} \underset{Zn}{\xrightarrow{H_2O}} \text{B}$
The compound B is
CH3COCH3
CH3CH2CHO
CH3CHO
CH3CH2COCH3
22680.The product formed in Aldol condensation is
a beta–hydroxy aldehyde or a beta–hydroxy ketone.
an alpha–hydroxy aldehyde or ketone.
an alpha, beta unsaturated ester
a beta–hydroxy acid
22681.Which of the following is correct?
Any aldehyde gives secondary alcohol on reduction
Reaction of vegetable oil with H2SO4 gives glycerin
C2H5OH, iodine with NaOH gives iodoform
Sucrose on reaction with NaCl give invert sugar
22682.When CH2=CH–O–CH2–CH3 reacts with one mole of HI, one of the products formed is
ethane
ethanol
iodoethene
ethanal
22683.Write IUPAC name of following compound
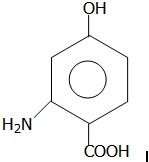

2–Amino–4–hydroxybenzoic acid
6–Amino–4–hydroxybenzoic acid
3–Amino–4–carboxyphenol
2–Carboxy–5–hydroxyaniline
22684.Benzene can be conveniently converted into n–propyl benzene by
Friedel – Craft alkylation with n–propyl chloride
Friedel – Craft acylation with propionyl chloride followed by Wolff – Kishner reduction
Friedel – Craft acylation with propionyl chloride followed by catalytic hydrogenation
Friedel – Craft acylation with propionyl chloride followed by reduction with LiAlH4
22685.The compound formed as a result of oxidation of ethyl benzene by KMnO4 is
Benzyl alcohol
Benzoic acid
Acetophenone
Benzophenone
22686.The compound which forms acetaldehyde when heated with dilute NaOH is
1 chloro ethane
1, 1 dichloro ethane
1, 2 dichloro ethane
1, 1, 1 trichloro ethane
22687.The correct order of decreasing acid strength of trichloroacetic acid (A), trifluoroacetic acid (B), acetic acid (C) and formic acid (D) is
A > B > C > D
A > C > B > D
B > A > D > C
B > D > C > A
22689.Compound 'A' undergoes formation of cyanohydrins which on hydrolysis gives lactic acid (CH3CHOHCOOH). Therefore, compound 'A' is
formaldehyde
acetaldehyde
acetone
benzaldehyde