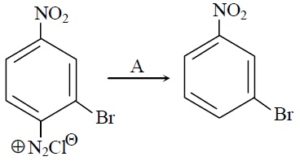
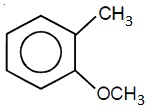
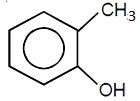
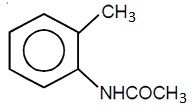
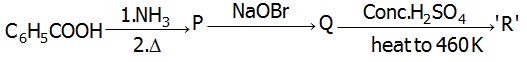22706.In the Hofmann bromamide degradation reaction, the number of moles of NaOH and Br2 used per mole of amine produced are
Four moles of NaOH and two moles of Br2
Two moles of NaOH and two moles of Br2
Four moles of NaOH and one mole of Br2
One mole of NaOH and one mole of Br2
22707.Diethyl amine when treated with nitrous acid yields
Diethyl ammonium nitrite
Ethyl alcohol
N–nitroso diethyl amine
Triethyl ammonium nitrite
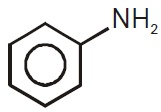
22711.The amine ‘A’ when treated with nitrous acid gives yellow oily substance. The amine A is
triethylamine
trimethylamine
aniline
methylphenylamine
22712.The reaction of aniline with chloroform under alkaline conditions leads to the formation of
Phenyl cyanide
Phenyl isonitrile
Phenyl cyanate
Phenyl isocyanate
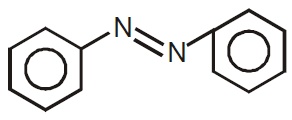
22713.Which of the following compound will not undergo azo coupling reaction with benzene diazonium chloride?
Aniline
Phenol
Anisole
Nitrobenzene
22714.In the chemical reactions,
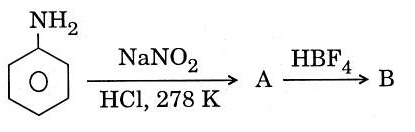
the compounds A and B respectively are
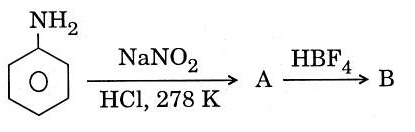
the compounds A and B respectively are
Nitrobenzene and chlorobenzene
Nitrobenzene and fluorobenzene
Phenol and benzene
Benzene diazonium chloride and fluorobenzene
22715.Which of the following statements about primary amines is false?
Alkyl amines are stronger bases than aryl amines
Alkyl amines react with nitrous acid to produce alcohols
Aryl amines react with nitrous acid to produce phenols
Alkyl amines are stronger bases than ammonia
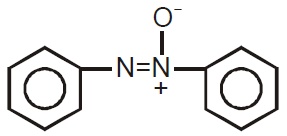
22716.Which of the following is the most stable diazonium salt ?
C6H5CH2N+
2X−
2X−
CH3N+
2X−
2X−
CH3CH2N+
2X−
2X−
C6H5N+
2X−
2X−
22717.Considering the basic strength of amines in aqueous solution, which one has the smallest pKb value?
CH3NH2
(CH3)3N
C6H5NH2
(CH3)2NH
22719.In the following sequence of reactions
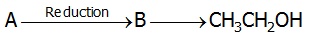 The compound A is
The compound A is
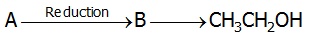 The compound A is
The compound A is
Propane nitrile
Ethane nitrile
CH3NO2
CH3NC
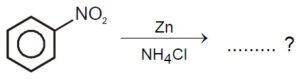
22720.Reduction of aromatic nitro compounds using Fe and HCl gives _____.
aromatic oxime
aromatic hydrocarbon
aromatic primary amine
aromatic amide
22721.The correct statement regarding the basicity of arylamines is :
Arylamines are generally more basic than alkylamines because of aryl group
Arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is sp–hybridized
Arylamines are generally less basic than alkylamines because the nitrogen lone–pair electrons are delocalized by interaction with the aromatic ring π electron system
Arylamines are generally more basic than alkylamines because the nitrogen lone–pair electrons are not delocalized by interaction with the aromatic ring π electron system
22722.One of the following amide will not undergo Hoffmann bromamide reaction:
CH3CONH2
CH3CONHCH3
C6H5CONH2
CH3CH2CONH2
22723.Which one of the following on reduction with lithium aluminium hydride yields a secondary amine?
Methyl isocyanide
Acetamide
Methyl cyanide
Nitroethane