13949.The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
Rate of decomposition is very slow
Rate is proportional to the surface coverage
Rate is inversely proportional to the surface coverage
Rate is independent of the surface coverage
Explanation:
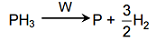

Rate = k[PH3]
It is dependent on surface coverage because at low pressure, surface area covered is proportional to partial pressure of PH3 and it is first order w.r.t PH3.
θ = $\dfrac{kP}{1 + kP}$
θ = fraction of surface area covered at low pressure
θ = k P
21201.The number of moles of KMnO4 that will be needed to react with one mole of sulphite ion in acidic solution is:
1
3/5
4/5
2/5
21236.The electronic configuration of an element is 1s2 2s2 2p6 3s2 3p6 3d5 4s1. This presentation is
excited state
ground state
cationic form
anionic form
21253.With increase in principal quantum number n, the energy difference between adjacent energy levels in hydrogen atom
increases
decreases
remain constant
decreases for lower values of n and increases for higher values of n
21311.The energy of second Bohr orbit of the hydrogen atom is –328 kJ mol–1; hence the energy of fourth Bohr orbit would be
–41 kJ mol–1
–1312 kJ mol–1
–164 kJ mol–1
–82 kJ mol–1
21354.Which one of the following constitutes a group of the isoelectronic species?
CO2–
2, O–
2, CO, NO
2, O–
2, CO, NO
NO+, C2–
2, CN–, N2
2, CN–, N2
CN–, N2, O2–
2, C2–
2
2, C2–
2
N2, O–
2, NO+, CO
2, NO+, CO
21359.Dipole-induced dipole interactions are present in which of the following pairs?
H2O and alcohol
Cl2 and CCl4
HCl and He atoms
SiF4 and He atoms
21376.Which of the following contains maximum number of lone pairs on the central atom?
ClO–
3
3
XeF4
SF4
I–
3
3
21445.In the equation of state of an ideal gas PV = nRT, the value of the universal gas constant would depend only on
the nature of the gas
the pressure of the gas
the units of the measurement
None of the above
21469.Based on the first law of thermodynamics, which one of the following is correct?
For an isothermal process, q = +w
For an isochoric process, ΔU = –q
For an adiabatic process, ΔU = –w
For a cyclic process, q = –w
21478.In a constant volume calorimeter, 3.5 g of a gas with molecular weight 28 was burnt in excess oxygen at 298.0 K. The temperature of the calorimeter was found to increase from 298.0 K to 298.45 K due to the combustion process. Given that the heat capacity of the calorimeter is 2.5 kJ K–1, the numerical value for the enthalpy of combustion of the gas in kJ mol–1 is
3
7
8
9
21591.Which of the following statements regarding ozone is not correct?
The oxygen–oxygen bond length in ozone is identical with that of molecular oxygen
The ozone is response hybrid of two structures
The ozone molecule is angular in shape
Ozone is used as a germicide and disinfectant for the purification of air.
21607.The function of Fe(OH)3 in the contact process is
to detect colloidal impurity
to remove moisture
to remove dust particles
to remove arsenic impurity
21609.Helium is used in balloons in place of hydrogen because it is
incombustible
more abundant than hydrogen
radioactive
lighter than hydrogen
21696.The hydrocarbon which can react with sodium in liquid ammonia is
CH3CH2CH2C≡CCH2CH2CH3
CH3CH2C≡CH
CH3CH=CHCH3
CH3CH2C≡CCH2CH3
21752.AB is an ionic solid. If the ratio of ionic radii of A+and B– is 0.52. What is the coordination number of B–?
6
3
2
8
22276.The rusting of iron takes place as follows
2H+ + 2e– + $\dfrac{1}{2}$O2 → H2O(l); E° = +1.23 V
Fe2+ + 2e– → Fe(s); E° = –0.44 V
Calculate ΔG° for the net process
–322 kJ mol–1
–161 kJ mol–1
–152 kJ mol–1
–76 kJ mol–1
22284.The equivalent conductances of two strong electrolytes at infinite dilution in H2O (where ions move freely through a solution) at 25°C are given below:
Λo
CH3COONa = 91.0 S cm2 / equiv
Λo
CHCl = 426.2 S cm2 / equiv
What additional information/quantity one needs to calculate Λ° of an aqueous solution of acetic acid?
Λ° of NaCl
Λ° of CH3COOK
The limiting equivalent conductance of H+ (λo
H+)
H+)
Λ° of chloroacetic acid (ClCH2COOH)
22287.In the electrolysis of acidulated water, it is desired to obtain 1.12 cc of hydrogen per second under S.T.P. conditions. The current to be passed is
0.965 Amp
19.3 Amp
9.65 Amp
1.93 Amp
22300.The reduction potential values of X, Y and Z are –3.05 V, –0.44 V and –0.83 V respectively. Which of the following order is correct with respect to their reducing property?
X > Y > Z
X > Z > Y
Y > Z > X
Z > Y > X
22372.For the decomposition of a compound AB at 600 K, the following data were obtained
The order for the decomposition of AB is
| [AB] mol dm–3 | Rate of decomposition of AB in mol dm–3 s–1 |
|---|---|
| 0.20 | 2.75 × 10–8 |
| 0.40 | 11.0 × 10–8 |
| 0.60 | 24.75 × 10–8 |
The order for the decomposition of AB is
0
1
2
1.5
22402.The rate of reaction between two reactants A and B decreases by a factor of 4 if the concentration of reactant B is doubled. The order of reaction with respect to reactant B is
2
–2
1
–1
22405.The concentration of R in the reaction R → P was measured as a function of time and the following data is obtained
The order of the reaction is
| [R] (molar) | 1.0 | 0.75 | 0.40 | 0.10 |
| t (min) | 0.0 | 0.05 | 0.12 | 0.18 |
The order of the reaction is
first
second
third
zero
22425.At given temperature and pressure adsorption of which gas of the following will take place the most?
Dihydrogen
Dioxygen
Ammonia
Dinitrogen
22444.Which of the following characteristics is not correct for physical adsorption?
Adsorption on solid is reversible.
Adsorption is spontaneous.
Adsorption increases with increase in temperature.
Both enthalpy and entropy of adsorption are negative.
22525.A magnetic moment of 1.73 BM will be shown by one among the following
[Cu(NH3)4]2+
[Ni(CN)4]2–
TiCl4
[CoCl6]4–
22568.Which of the following pairs represents linkage isomers?
[Pd(PPh3)2(NCS)2] and [Pd(PPh3)2(SCN)2]
[Co(NH3)5NO3]SO4 and [Co(NH3)5SO4]NO3
[PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2
[Cu(NH3)4][PtCl4] and [Pt(NH3)4][CuCl4]
22577.Chlorobenzene is formed by reaction of chlorine with benzene in the presence of AlCl3. Which of the following species attacks the benzene ring in this reaction?
Cl–
Cl+
AlCl3
[AlCl4]–
22604.The conversion of m–nitrophenol to resorcinol involves respectively
diazotization, reduction and hydrolysis
hydrolysis, diazotization and reduction
reduction, diazotization and hydrolysis
hydrolysis, reduction and diazotization
22621.Phenol can be converted to o–hydroxybenzaldehyde by
Kolbe's reaction
Reimer–Tiemann reaction
Wurtz reaction
Sandmeyer's reaction
22634.An organic compound A containing C, H and O has a pleasant odour with boiling point of 78°C. On boiling A with concentrated H2SO4, a colourless gas is produced which decolourises bromine water and alkaline KMnO4. The organic liquid A is
C2H5COOCH3
C2H5OH
C2H5Cl
C2H6
22702.Benzaldehyde and acetone can be best distinguished using
hydrazine
Tollen's reagent
sodium hydroxide solution
2, 4–DNP
22714.In the chemical reactions,
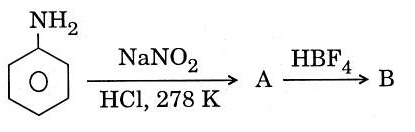
the compounds A and B respectively are
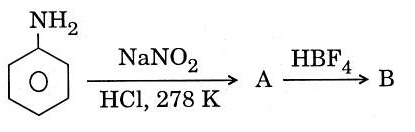
the compounds A and B respectively are
Nitrobenzene and chlorobenzene
Nitrobenzene and fluorobenzene
Phenol and benzene
Benzene diazonium chloride and fluorobenzene
22809.The monomers of Buna–S rubber are
vinyl chloride and sulphur
butadiene
styrene and butadiene
isoprene and butadiene
22814.Given the polymers: A = Nylon; B = Buna–S; C = Polythene. Arrange these in decreasing order of their intermolecular forces.
A > B > C
B > C > A
B < C < A
C < A < B
22828.Structure of some common polymers are given. Which one is not correctly presented?
Nylon 66 [— NH(CH2 )6NHCO(CH2)4 — CO —]2
Teflon (— CF2 — CF2 — )n
Neoprene 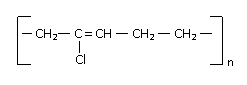
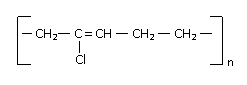
Terylene 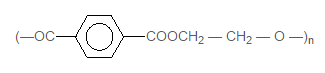
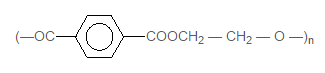
22855.Which is used in formation of nylon–66?
Sulphur haxafluoride
Adipic acid
Sulphurous acid
Phthalic acid
22863.Biodegradable polymer which can be produced from glycine and aminocaproic acid is
Nylon 2 – nylon 6
PHBV
Buna – N
Nylon 6, 6
22922.Which one of the following is not a target molecule for drug function in body?
Vitamins
Carbohydrates
Lipids
Proteins
