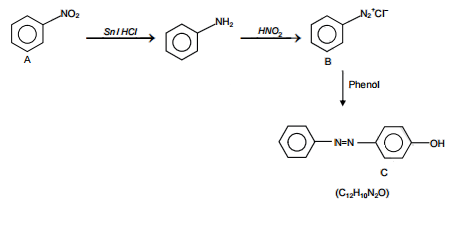
A given nitrogen-containing aromatic compound A reacts with Sn/HCl, followed by HNO2 to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is
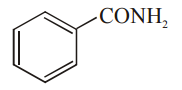
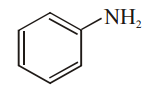
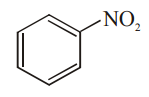
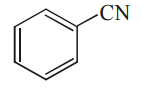
Explanation: