The compound that will react most readily with gaseous bromine has the formula:
C2H4
C3H6
C2H2
C4H10
Explanation:
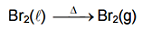
Bromine naturally exist in liquid phase and in question bromine is given in gaseous phase.
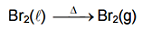
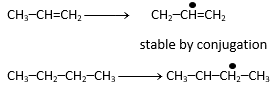
Reaction takes place at high temperature so mechanism with Br2(g) should be free radical substitution which can take place in propene and butane.
Rate of free radical substitution reaction depend upon stability of free radical.

Propenyl free radical is more stable so answer will be C3H6
