The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
Rate of decomposition is very slow
Rate is proportional to the surface coverage
Rate is inversely proportional to the surface coverage
Rate is independent of the surface coverage
Explanation:
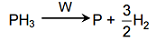

Rate = k[PH3]
It is dependent on surface coverage because at low pressure, surface area covered is proportional to partial pressure of PH3 and it is first order w.r.t PH3.
θ = $\dfrac{kP}{1 + kP}$
θ = fraction of surface area covered at low pressure
θ = k P
