During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is
330 minutes
55 minutes
110 minutes
220 minutes
Explanation:
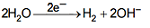
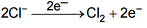
At Cathode:
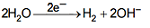
At anode:
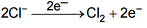
$\dfrac{W}{E} = \dfrac{It}{96500}$
0.1 × 2 = $\dfrac{3 × t(sec)}{96500}$
t = 6433 sec
t = 107.2 min
∼ 110 min.
