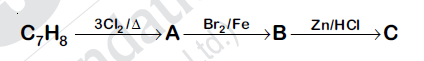|
Nitration of aniline in strong acidic medium also gives m-nitroaniline because
|
Answer
|
|
The difference between amylose and amylopectin is
|
Answer
|
|
A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with conc. $H_2SO_4$. The evolved gaseous mixture is passed through KOH pellets. Weight (in g) of the remaining product at STP will be
|
Answer
|
|
Which of the following oxides is most acidic in nature?
|
Answer
|
|
Which oxide of nitrogen is not a common pollutant introduced into the atmosphere both due to natural and human activity?
|
Answer
|
|
The compound A on treatment with Na gives B, and with $PCl_5$ gives C. B and C react together to give diethyl ether. A, B and C are in the order
|
Answer
|
|
The compound $C_7H_8$ undergoes the following reactions:
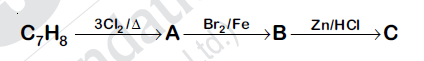
The product C is
|
Answer
|
|
Hydrocarbon (A) reacts with bromine by substitution to form an alkyl bromide which by Wurtz reaction is converted to gaseous hydrocarbon containing less than four carbon atoms. (A) is
|
Answer
|
|
Which of the following molecules represents the order of hybridisation $sp^2, sp^2$, sp, sp from left to right atoms?
|
Answer
|
|
Which of the following carbocations is expected to be most stable?
|
Answer
|