22735.Aniline in a set of the following reactions yielded a coloured product ‘Y’
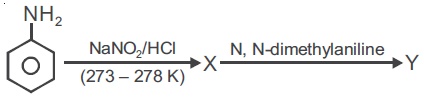
The structure of Y would be :

The structure of Y would be :
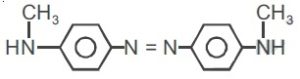
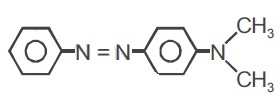
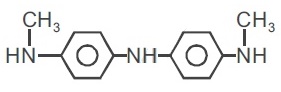
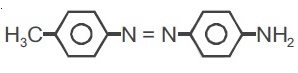
22736.The reagent with which the following reaction is best accomplished is
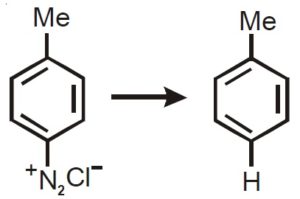

H3PO2
H3PO3
H3PO4
NaHSO3
22737.In the chemical reaction,
CH3CH2NH2 + CHCl3 + 3KOH → (A) + (B) + 3H2O
the compounds (A) and (B) are respectively
C2H5CN and 3KCl
CH3CH2CONH2 and 3KCl
C2H5NC and K2CO3
C2H5NC and 3KCl
22738.In the given set of reactions,
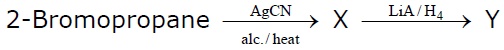
the IUPAC name of product ‘Y’ is
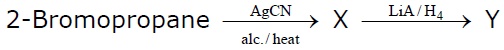
the IUPAC name of product ‘Y’ is
N–Methylpropanamine
N–Isopropylmethylamine
Butan–2–amine
N–Methylpropan–2–amine
22739.On heating an aliphatic primary amine with chloroform and ethanolic potassium hydroxide, the organic compound formed is
An alkanediol
An alkyl cyanide
An alkyl isocyanide
An alkanol
22740.Method by which Aniline cannot be prepared is
Potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution
Hydrolysis of phenylisocyanide with acidic solution
Degradation of benzamide with bromine in alkaline solution
Reduction of nitrobenzene with H2/Pd in ethanol
22741.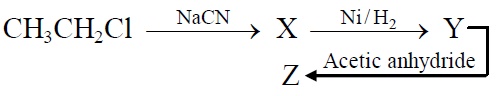
Z in the above reaction sequence is:
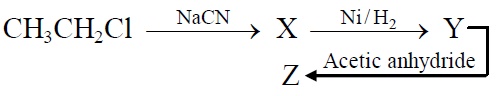
Z in the above reaction sequence is:
CH3CH2CH2NHCOCH3
CH3CH2CH2NH2
CH3CH2CH2CONHCH3
CH3CH2CH2CONHCOCH3
22742.Select the compound which on treatment with nitrous acid liberates nitrogen.
Nitroethane
Triethylamine
Diethylamine
Ethylamine
22743.Toluene is nitrated and the resulting product is reduced with tin and hydrochloric acid. The product so obtained is diazotised and then heated with cuprous bromide. The reaction mixture so formed contains
mixture of o− and p−bromotoluenes
mixture of o− and p−dibromobenzenes
mixture of o− and p−bromoanilines
mixture of o− and m−bromotoluenes
22744.Primary nitroalkanes are obtained in good yield by oxidising aldoximes with the help of
trifluoroperoxyacetic acid
acidified potassium permanganate
concentrated nitric acid
potassium dichromate and dilute sulphuric acid
