21733.The coordination number of a metal crystallizing in a hexagonal close packing (hcp) structure is
12
8
6
7
21734.The packing efficiency of the two dimensional square unit cell shown is
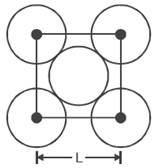

39.27%
68.02%
74.05%
78.54%
21735.AB crystallizes in a body centred cubic lattice with edge length 'a' equal to 387 pm. The distance between two opposively charged ions in the lattice is
200 pm
300 pm
335 pm
250 pm
21736.A compound contains two types of atoms X and Y. It crystallizes in a cubic lattice with atoms X at the corners of the unit cell and atoms Y at the body centre. The simplest possible formula of this compound is:
X8Y
X2Y
XY
XY8
21737.A compound of 'A' and 'B' crystallizes in a cubic lattice in which the 'A' atoms occupy the lattice points at the corners of the cube. The 'B' atoms occupy the centre of each face of the cube. The probable empirical formula of the compound is
AB3
AB
A3B
AB2
21738.Copper crystallises in a face–centred cubic lattice with a unit cell length of 361 pm. What is the radius of copper atom in pm?
108
181
157
128
21739.In a solid 'AB' having NaCl structure, 'A' atoms occupy the corners of the cubic unit cell. If all the face–centred atoms along one of the axes are removed, then the resultant stoichiometry of the solid is
AB2
A2B
A4B3
A3B4
21740.A metal crystallizes with a face–centered cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
144 pm
204 pm
288 pm
408 pm
21741.How many unit cells are present in a cube–shaped ideal crystal of NaCl of mass 1.0 g?
5.14 × 1021 unit cells
1.28 × 1021 unit cells
1.71 × 1021 unit cells
2.57 × 1021 unit cells
21742.A metallic crystal having bcc type staking pattern, what percentage of volume of this lattice is empty space?
68%
32%
26%
74%
21743.A p–type materialis electrically
positive
negative
neutral
depends upon the concentration of p–impurities
21744.The number of tetrahedral voids in the unit cell of a face centred cubic lattice of similar atoms is
4
6
8
10
21745.In a triclinic crystal:
a = b = c, α = β = γ ≠ 90°
a ≠ b = c, α = β = γ = 90°
a ≠ b ≠ c, α ≠ β ≠ γ ≠ 90°
a ≠ b ≠ c, α = γ = 90° ≠ β
21746.A solid compound contains X, Y and Z atoms in a cubic lattice with X atoms occupying the corners, Y atoms in the body centred position and Z atoms at the centres of faces of the unit cell. What is the empirical formula of the compound?
XY2Z3
XYZ3
X2Y2Z3
X8YZ6
21747.The fraction of total volume occupied by the atoms present in a simple cube is
$\dfrac{\pi}{3\sqrt{2}}$
$\dfrac{\pi}{4\sqrt{2}}$
$\dfrac{\pi}{4}$
$\dfrac{\pi}{6}$
21748.Percentage of free space in cubic close packed structure and in body centred packed structure are respectively
30% and 26%
48% and 26%
32% and 48%
26% and 32%
21750.A substance AxBy crystallizes in a face centred cubic (fcc) lattice in which atoms 'a' occupy each corner of the cube and atoms ‘B’ occupy the centres of each face of the cube. Identify the correct composition of the substance AxBy:
AB3
A4B3
A3B
Composition cannot be specified
21751.Which one of the following compound exhibits both Schottky and Frenkel defects?
NaCl
AgCl
AgBr
AgI
21752.AB is an ionic solid. If the ratio of ionic radii of A+and B– is 0.52. What is the coordination number of B–?
6
3
2
8
