AIF3 is soluble in HF only in presence of KF. It is due to the formation of
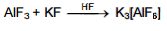
maximum C.N. of Al+3 is six so it forms $AlF_6^{3–}$

AIF3 is soluble in HF only in presence of KF. It is due to the formation of

maximum C.N. of Al+3 is six so it forms $AlF_6^{3–}$
|
Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because |
Answer |
|
The suspension of slaked lime in water is known as |
Answer |
|
The hybridizations of atomic orbitals of nitrogen in NO+ |
Answer |
|
Which of the following fluoro-compounds is most likely to behave as a Lewis base? |
Answer |
|
Which of the following pairs of ions is isoelectronic and isostructural? |
Answer |
|
In context with beryllium, which one of the following statements is incorrect? |
Answer |
|
Hot concentrated sulphuric acid is a moderately strong oxidizing agent. Which of the following reactions does not show oxidizing behaviour? |
Answer |
|
Which of the following pairs of d-orbitals will have electron density along the axes? |
Answer |
|
The correct geometry and hybridization for XeF4 are – |
Answer |
|
Among the following, which one is a wrong statement? |
Answer |