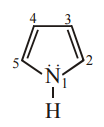
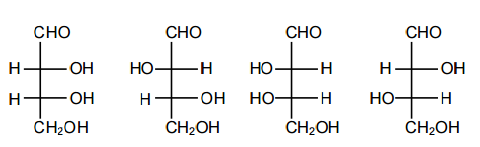
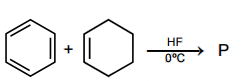
Which one of the following statements related to lanthanons is incorrect?
Ce+4 is strong oxidising agent and easily convert into Ce+3
Eu+2 exist and behave as reducing agent.
Ln(OH)3 are basic in nature and as ionic radius decrease, basic nature decreases from Ce to Lu.
Whether all lanthanons are much more reactive than aluminum can’t be said.