In which of the following molecules, all atoms are coplanar?
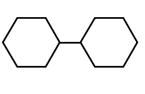
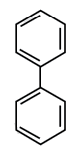
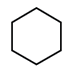
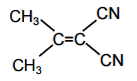
All carbon atom is sp2 hybridised and its geometry is trigonal planar.

In which of the following molecules, all atoms are coplanar?





All carbon atom is sp2 hybridised and its geometry is trigonal planar.
|
Which one of the following structures represents nylon 6,6 polymer? |
Answer |
|
In pyrrole |
Answer |
|
Which of the following compounds shall not produce propene by reaction with HBr followed by elimination or direct only elimination reaction ? |
Answer |
|
Which one of the following nitro-compounds does not react with nitrous acid |
Answer |
|
The central dogma of molecular genetics states that the genetic information flows from |
Answer |
|
The correct corresponding order of names of four aldoses with configuration given below |
Answer |
|
In the given reaction |
Answer |
|
A given nitrogen-containing aromatic compound A reacts with Sn/HCl, followed by HNO2 to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is |
Answer |
|
Consider the reaction |
Answer |
|
The correct structure of the product A formed in the reaction |
Answer |