|
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
|
Answer
|
|
The correct order of atomic radii in group 13 elements is
|
Answer
|
|
Which of the following statements is not true for halogens?
|
Answer
|
|
In the structure of $ClF_3$, the number of lone pair of electrons on central atom ‘Cl’ is
|
Answer
|
|
Identify the major products P, Q and R in the following sequence of reactions:
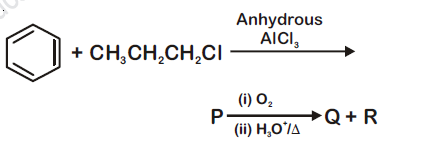
|
Answer
|
|
Which of the following compounds can form a zwitterion?
|
Answer
|
|
Regarding cross-linked or network polymers, which of the following statements is incorrect?
|
Answer
|
|
Nitration of aniline in strong acidic medium also gives m-nitroaniline because
|
Answer
|
|
The difference between amylose and amylopectin is
|
Answer
|
|
A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with conc. $H_2SO_4$. The evolved gaseous mixture is passed through KOH pellets. Weight (in g) of the remaining product at STP will be
|
Answer
|