|
On which of the following properties does the coagulating power of an ion depend?
|
Answer
|
|
Given van der Waals constant for $NH_3, H_2, O_2$ and $CO_2$ are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied?
|
Answer
|
|
The solubility of $BaSO_4$ in water is 2.42 × $10^{–3} gL^{–1}$ at 298 K. The value of its solubility product $(K_{sp})$ will be
(Given molar mass of
$BaSO_4 = 233 g mol–1)$
|
Answer
|
|
In which case is number of molecules of water maximum?
|
Answer
|
|
The correct difference between first and second order reactions is that
|
Answer
|
|
Among $CaH_2, BeH_2 , BaH_2,$ the order of ionic character is
|
Answer
|
|
Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below :
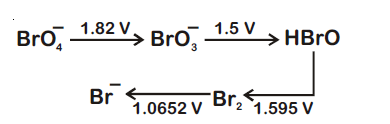
Then the species undergoing disproportionation is
|
Answer
|
|
For the redox reaction
$MnO_4^-+C_2O_4^{2-}+H^+\rightarrow Mn^{2+} +CO_2 + H_2O$
The correct coefficients of the reactants for the balanced equation are
$MnO_4^-$ $C_2O_4^{2-}$ $H^+$
|
Answer
|
|
Which one of the following conditions will favour maximum formation of the product in the reaction,
$A_2(g)+B_2(g)\rightleftharpoons X_2(g)\triangle_r H=-XKJ?$
|
Answer
|
|
When initial concentration of the reactant is doubled, the half-life period of a zero order reaction
|
Answer
|