|
The correct order of N-compounds in its decreasing order of oxidation states is
|
Answer
|
|
Which one of the following elements is unable to form $MF_6^{3-}$ ion?
|
Answer
|
|
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
|
Answer
|
|
The correct order of atomic radii in group 13 elements is
|
Answer
|
|
Which of the following statements is not true for halogens?
|
Answer
|
|
In the structure of $ClF_3$, the number of lone pair of electrons on central atom ‘Cl’ is
|
Answer
|
|
Identify the major products P, Q and R in the following sequence of reactions:
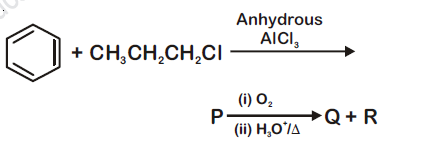
|
Answer
|
|
Which of the following compounds can form a zwitterion?
|
Answer
|
|
Regarding cross-linked or network polymers, which of the following statements is incorrect?
|
Answer
|
|
Nitration of aniline in strong acidic medium also gives m-nitroaniline because
|
Answer
|