In the reaction
The electrophile involved is
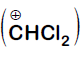
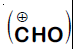
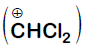
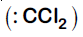
It is Reimer-Tiemann reaction. The electrophile formed is $:CCl_2$ (Dichlorocarbene) according to the following reaction

In the reaction
The electrophile involved is
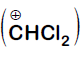
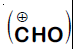
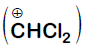
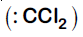
|
Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their |
Answer | ||||||||||||
|
Compound $A, C_8H_{10}O$, is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell. |
Answer | ||||||||||||
|
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
a b c d |
Answer | ||||||||||||
|
Which one of the following ions exhibits d-d transition and paramagnetism as well? |
Answer | ||||||||||||
|
Iron carbonyl, $Fe(CO)_5$ is |
Answer | ||||||||||||
|
The type of isomerism shown by the complex $[CoCl_2(en)_2]$ is |
Answer | ||||||||||||
|
The geometry and magnetic behaviour of the complex $[Ni(CO)_4]$ are |
Answer | ||||||||||||
|
Following solutions were prepared by mixing different volumes of NaOH and HCl of different concentrations : |
Answer | ||||||||||||
|
On which of the following properties does the coagulating power of an ion depend? |
Answer | ||||||||||||
|
Given van der Waals constant for $NH_3, H_2, O_2$ and $CO_2$ are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied? |
Answer |