|
The type of isomerism shown by the complex $[CoCl_2(en)_2]$ is
|
Answer
|
|
The geometry and magnetic behaviour of the complex $[Ni(CO)_4]$ are
|
Answer
|
|
Following solutions were prepared by mixing different volumes of NaOH and HCl of different concentrations :
a. $60 mL\dfrac{M}{10}HCL+40 mL\dfrac{M}{10} NaOH$
b. $55 mL\dfrac{M}{10}HCL+45 mL\dfrac{M}{10} NaOH$
c. $75 mL\dfrac{M}{5}HCL+25 mL\dfrac{M}{5} NaOH$
d. $100 mL\dfrac{M}{10}HCL+100 mL\dfrac{M}{10} NaOH$
pH of which one of them will be equal to 1?
|
Answer
|
|
On which of the following properties does the coagulating power of an ion depend?
|
Answer
|
|
Given van der Waals constant for $NH_3, H_2, O_2$ and $CO_2$ are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied?
|
Answer
|
|
The solubility of $BaSO_4$ in water is 2.42 × $10^{–3} gL^{–1}$ at 298 K. The value of its solubility product $(K_{sp})$ will be
(Given molar mass of
$BaSO_4 = 233 g mol–1)$
|
Answer
|
|
In which case is number of molecules of water maximum?
|
Answer
|
|
The correct difference between first and second order reactions is that
|
Answer
|
|
Among $CaH_2, BeH_2 , BaH_2,$ the order of ionic character is
|
Answer
|
|
Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below :
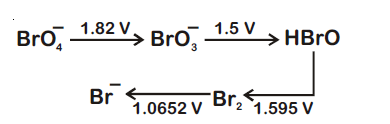
Then the species undergoing disproportionation is
|
Answer
|