44827.The compound $C_7H_8$ undergoes the following reactions:
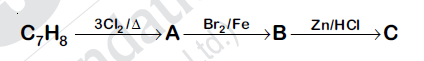
The product C is
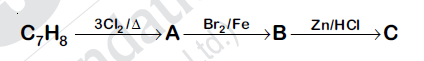
The product C is
3-bromo-2,4,6-trichlorotoluene
o-bromotoluene
m-bromotoluene
p-bromotoluene
Explanation:

So, the correct option is (3)
So, the correct option is (3)
44828.Hydrocarbon (A) reacts with bromine by substitution to form an alkyl bromide which by Wurtz reaction is converted to gaseous hydrocarbon containing less than four carbon atoms. (A) is
$CH_3– CH_3$
$CH_2 = CH_2$
$CH \equiv CH $
$CH_4$
Explanation:

Hence the correct option is (4)
Hence the correct option is (4)
44829.Which of the following molecules represents the order of hybridisation $sp^2, sp^2$, sp, sp from left to right atoms?
$CH_2= CH – CH = CH_2$
$CH_2= CH – C \equiv CH$
$HC \equiv C – C \equiv CH$
$CH_3– CH = CH – CH_3$
Explanation:

Number of orbital require in hybridization= Number of $\sigma$-bonds around each carbon atom.
Number of orbital require in hybridization= Number of $\sigma$-bonds around each carbon atom.
44830.Which of the following carbocations is expected to be most stable?
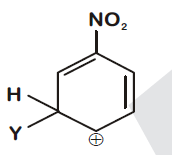
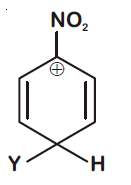
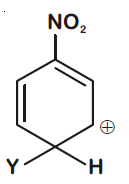
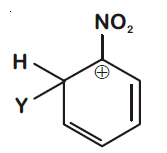
Explanation:
$–NO_2$ group exhibit –I effect and it decreases with increase in distance. In option (1) positive charge present on C-atom at maximum distance so –I effect reaching to it is minimum and stability is maximum.
$–NO_2$ group exhibit –I effect and it decreases with increase in distance. In option (1) positive charge present on C-atom at maximum distance so –I effect reaching to it is minimum and stability is maximum.
44831.Which of the following is correct with respect to – I effect of the substituents? (R = alkyl)
$– NH_2 > – OR > – F$
$– NR_2 < – OR < – F$
$– NH_2 < – OR < – F$
$– NR_2 > – OR > – F$
Explanation:
–I effect increases on increasing electronegativity of atom. So, correct order of –I effect is $–NH_2$ < – OR < – F. *Most appropriate Answer is option (3), however option (2) may also be correct answer.
–I effect increases on increasing electronegativity of atom. So, correct order of –I effect is $–NH_2$ < – OR < – F. *Most appropriate Answer is option (3), however option (2) may also be correct answer.
44832.In the reaction
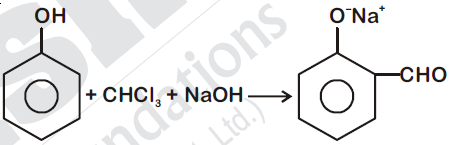
The electrophile involved is

The electrophile involved is
Dichloromethyl anion 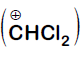
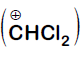
Formyl cation 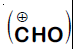
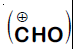
Dichloromethyl cation 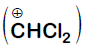
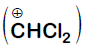
Dichlorocarbene 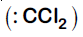
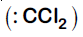
Explanation:
It is Reimer-Tiemann reaction. The electrophile formed is $:CCl_2$ (Dichlorocarbene) according to the following reaction

It is Reimer-Tiemann reaction. The electrophile formed is $:CCl_2$ (Dichlorocarbene) according to the following reaction
44833.Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their
More extensive association of carboxylic acid via van der Waals force of attraction
Formation of carboxylate ion
Formation of intramolecular H-bonding
Formation of intermolecular H-bonding
Explanation:
Due to formation of intermolecular H-bonding in carboxylic acid, association occurs. Hence boiling point increases and become more than the boiling point of aldehydes, ketones and alcohols of comparable molecular masses.
Due to formation of intermolecular H-bonding in carboxylic acid, association occurs. Hence boiling point increases and become more than the boiling point of aldehydes, ketones and alcohols of comparable molecular masses.
44834.Compound $A, C_8H_{10}O$, is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell.
A and Y are respectively
A and Y are respectively
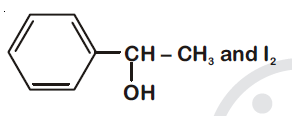
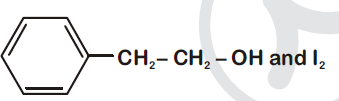
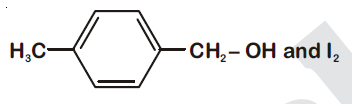
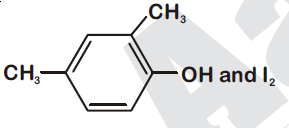
Explanation:
Option (1) is secondary alcohol which on oxidation gives phenylmethyl ketone (Acetophenone). This on reaction with $I_2$ and NaOH form iodoform and sodium benzoate.

Option (1) is secondary alcohol which on oxidation gives phenylmethyl ketone (Acetophenone). This on reaction with $I_2$ and NaOH form iodoform and sodium benzoate.
44835.Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
a b c d
| Column I | Column II |
|---|---|
| a. $Co^{3+}$ | i. $\sqrt{8}BM$ |
| b. $Cr^{3+}$ | ii. $\sqrt{35}BM$ |
| c. $Fe^{3+}$ | iii. $\sqrt{3}BM$ |
| d. $Ni^{2+}$ | iv. $sqrt{24}BM$ |
| v. $sqrt{15}BM$ |
a b c d
iv i ii iii
i ii iii iv
iv v ii i
iii v i ii
Explanation:
$Co^{3+} = [Ar] 3d^6, Unpaired e^–(n) = 4$
Spin magnetic moment = $\sqrt{4(4 +2)}=\sqrt{24} BM$
$\left[Ar\right]3d^3$, Unpaired e^–(n) = 3
Spin magnetic moment = $\sqrt{3(3 +2)}=\sqrt{15} BM$
$Fe^{3+}=\left[Ar\right]3d^5,$ Unpaired $e^{-}(n)=5$
Spin magnetic moment =$\sqrt{5(5 +2)}=\sqrt{35} BM$
$Ni^{2+} = [Ar] 3d^8,$ Unpaired $e^{-}(n)=2$
$\sqrt{2(2 +2)}=\sqrt{8}BM$
$Co^{3+} = [Ar] 3d^6, Unpaired e^–(n) = 4$
Spin magnetic moment = $\sqrt{4(4 +2)}=\sqrt{24} BM$
$\left[Ar\right]3d^3$, Unpaired e^–(n) = 3
Spin magnetic moment = $\sqrt{3(3 +2)}=\sqrt{15} BM$
$Fe^{3+}=\left[Ar\right]3d^5,$ Unpaired $e^{-}(n)=5$
Spin magnetic moment =$\sqrt{5(5 +2)}=\sqrt{35} BM$
$Ni^{2+} = [Ar] 3d^8,$ Unpaired $e^{-}(n)=2$
$\sqrt{2(2 +2)}=\sqrt{8}BM$
44836.Which one of the following ions exhibits d-d transition and paramagnetism as well?
$MnO_4^-$
$Cr_2O_7^{2–}$
$CrO_4^{2–}$
$MnO_4^{2–}$
Explanation:
$CrO_4^{2-}\Rightarrow Cr^{6+}=\left[Ar\right]$
Unpaired electron (n) = 0; Diamagnetic
$Cr_2O_7^{2-}\Rightarrow Cr^{6+}=\left[Ar\right]$
Unpaired electron (n) = 0; Diamagnetic
$MnO_4^{2-}=Mn^{6+}=\left[Ar\right]3d^1$
Unpaired electron (n) = 1; Paramagnetic
$MnO_4^-=Mn^{7+}=\left[Ar\right]$
Unpaired electron (n) = 0; Diamagnetic
$CrO_4^{2-}\Rightarrow Cr^{6+}=\left[Ar\right]$
Unpaired electron (n) = 0; Diamagnetic
$Cr_2O_7^{2-}\Rightarrow Cr^{6+}=\left[Ar\right]$
Unpaired electron (n) = 0; Diamagnetic
$MnO_4^{2-}=Mn^{6+}=\left[Ar\right]3d^1$
Unpaired electron (n) = 1; Paramagnetic
$MnO_4^-=Mn^{7+}=\left[Ar\right]$
Unpaired electron (n) = 0; Diamagnetic
44837.Iron carbonyl, $Fe(CO)_5$ is
Trinuclear
Mononuclear
Tetranuclear
Dinuclear
Explanation:
Based on the number of metal atoms present in a complex, they are classified into mononuclear, dinuclear, trinuclear and so on.
eg: $Fe(CO)_5$ : mononuclear
$Co_2(CO)_8$ : dinuclear
$Fe_3(CO)_{12}$ : trinuclear
Hence, option (2) should be the right answer.
Based on the number of metal atoms present in a complex, they are classified into mononuclear, dinuclear, trinuclear and so on.
eg: $Fe(CO)_5$ : mononuclear
$Co_2(CO)_8$ : dinuclear
$Fe_3(CO)_{12}$ : trinuclear
Hence, option (2) should be the right answer.
44838.The type of isomerism shown by the complex $[CoCl_2(en)_2]$ is
Ionization isomerism
Coordination isomerism
Geometrical isomerism
Linkage isomerism
Explanation:
In $[CoCl_2(en)_2]$, Coordination number of Co is 6 and this compound has octahedral geometry.

• As per given option, type of isomerism is geometrical isomerism.
In $[CoCl_2(en)_2]$, Coordination number of Co is 6 and this compound has octahedral geometry.
• As per given option, type of isomerism is geometrical isomerism.
44839.The geometry and magnetic behaviour of the complex $[Ni(CO)_4]$ are
Square planar geometry and paramagnetic
Tetrahedral geometry and diamagnetic
Square planar geometry and diamagnetic
Tetrahedral geometry and paramagnetic
Explanation:
$Ni(28) : [Ar]3d^8 4s^2$
∵CO is a strong field ligand
Configuration would be :

For, four ‘CO’-ligands hybridisation would be $sp^3$ and thus the complex would be diamagnetic and of tetrahedral geometry.

$Ni(28) : [Ar]3d^8 4s^2$
∵CO is a strong field ligand
Configuration would be :
For, four ‘CO’-ligands hybridisation would be $sp^3$ and thus the complex would be diamagnetic and of tetrahedral geometry.
44840.Following solutions were prepared by mixing different volumes of NaOH and HCl of different concentrations :
a. $60 mL\dfrac{M}{10}HCL+40 mL\dfrac{M}{10} NaOH$
b. $55 mL\dfrac{M}{10}HCL+45 mL\dfrac{M}{10} NaOH$
c. $75 mL\dfrac{M}{5}HCL+25 mL\dfrac{M}{5} NaOH$
d. $100 mL\dfrac{M}{10}HCL+100 mL\dfrac{M}{10} NaOH$
pH of which one of them will be equal to 1?
a. $60 mL\dfrac{M}{10}HCL+40 mL\dfrac{M}{10} NaOH$
b. $55 mL\dfrac{M}{10}HCL+45 mL\dfrac{M}{10} NaOH$
c. $75 mL\dfrac{M}{5}HCL+25 mL\dfrac{M}{5} NaOH$
d. $100 mL\dfrac{M}{10}HCL+100 mL\dfrac{M}{10} NaOH$
pH of which one of them will be equal to 1?
d
a
b
c
Explanation:
• Meq of HCl = $75\times\dfrac{1}{5}\times1=15$
• Meq of NaOH = $25\times\dfrac{1}{5}\times1=5$
• Meq of HCl in resulting solution = 10
• Molarity of $[H^+]$ in resulting mixture
$\dfrac{10}{100}=\dfrac{1}{10}$
$pH=-log\left[H^+\right]=log \left[\dfrac{1}{10}\right]=1.0$
• Meq of HCl = $75\times\dfrac{1}{5}\times1=15$
• Meq of NaOH = $25\times\dfrac{1}{5}\times1=5$
• Meq of HCl in resulting solution = 10
• Molarity of $[H^+]$ in resulting mixture
$\dfrac{10}{100}=\dfrac{1}{10}$
$pH=-log\left[H^+\right]=log \left[\dfrac{1}{10}\right]=1.0$
44841.On which of the following properties does the coagulating power of an ion depend?
Both magnitude and sign of the charge on the ion
Size of the ion alone
The magnitude of the charge on the ion alone
The sign of charge on the ion alone
Explanation:
• Coagulation of colloidal solution by using an electrolyte depends on the charge present (positive or negative) on colloidal particles as well as on its size.
• Coagulating power of an electrolyte depends on the magnitude of charge present on effective ion of electrolyte.
• Coagulation of colloidal solution by using an electrolyte depends on the charge present (positive or negative) on colloidal particles as well as on its size.
• Coagulating power of an electrolyte depends on the magnitude of charge present on effective ion of electrolyte.
44842.Given van der Waals constant for $NH_3, H_2, O_2$ and $CO_2$ are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied?
$O_2$
$H_2$
$NH_3$
$CO_2$
Explanation:
• van der waal constant ‘a’, signifies intermolecular forces of attraction.
• Higher is the value of ‘a’, easier will be the liquefaction of gas.
• van der waal constant ‘a’, signifies intermolecular forces of attraction.
• Higher is the value of ‘a’, easier will be the liquefaction of gas.
44843.The solubility of $BaSO_4$ in water is 2.42 × $10^{–3} gL^{–1}$ at 298 K. The value of its solubility product $(K_{sp})$ will be
(Given molar mass of $BaSO_4 = 233 g mol–1)$
(Given molar mass of $BaSO_4 = 233 g mol–1)$
$1.08 × 10^{–14} mol^2L^{–2}$
$1.08 × 10^{–12} mol^2L^{–2}$
$1.08 × 10^{–10} mol^2L^{–2}$
$1.08 × 10^{–8} mol^2L^{–2}$
Explanation:
Solubility of $BaSO_4, s =\dfrac{2.42 \times 10^{-3}}{233} (mol L^{-1})$
=$1.04 \times 10^{-5}(mol L^{-1})$

$K_{sp}=\left[Ba^{2+}\right]\left[SO_4^{2-}\right]= s^2$
$(1.04 \times 10^{-5})^2$
$1.08 \times 10^{-10} mol^2 L^{-2}$
Solubility of $BaSO_4, s =\dfrac{2.42 \times 10^{-3}}{233} (mol L^{-1})$
=$1.04 \times 10^{-5}(mol L^{-1})$
$K_{sp}=\left[Ba^{2+}\right]\left[SO_4^{2-}\right]= s^2$
$(1.04 \times 10^{-5})^2$
$1.08 \times 10^{-10} mol^2 L^{-2}$
44844.In which case is number of molecules of water maximum?
0.00224 L of water vapours at 1 atm and 273 K
0.18 g of water
18 mL of water
$10^{–3}$ mol of water
Explanation:
(1) Moles of water =$\dfrac{0.00224}{22.4}=10^{-4}$
Molecules of water = $mole \times N_{A}=10^{-4} N_A$
(2) Molecules of water = $mole × N_A =\dfrac{0.18}{18}N_A$
$10^{-2}N_A$
(3) Mass of water = 18 × 1 = 18 g
Molecules of water = mole × $N_A =\dfrac{18}{18}N_A$
$=N_A$
(4) Molecules of water = mole × $N_A = 10^{-3}N_A$
(1) Moles of water =$\dfrac{0.00224}{22.4}=10^{-4}$
Molecules of water = $mole \times N_{A}=10^{-4} N_A$
(2) Molecules of water = $mole × N_A =\dfrac{0.18}{18}N_A$
$10^{-2}N_A$
(3) Mass of water = 18 × 1 = 18 g
Molecules of water = mole × $N_A =\dfrac{18}{18}N_A$
$=N_A$
(4) Molecules of water = mole × $N_A = 10^{-3}N_A$
44845.The correct difference between first and second order reactions is that
A first-order reaction can catalyzed; a second-order reaction cannot be catalyzed
The half-life of a first-order reaction does not depend on $[A]_0$ ; the half-life of a second-order reaction does depend on $[A]_0$
The rate of a first-order reaction does not depend on reactant concentrations; the rate of a second-order reaction does depend on reactant concentrations
The rate of a first-order reaction does depend on reactant concentrations; the rate of a second-order reaction does not depend on reactant concentrations
Explanation:
• For first order reaction, $t_{1/2}=\dfrac{0.693}{k},$ which is independent of initial concentration of reactant.
• For second order reaction, $t_{1/2}=\dfrac{1}{k[A_0]},$ which depends on initial concentration of reactant.
• For first order reaction, $t_{1/2}=\dfrac{0.693}{k},$ which is independent of initial concentration of reactant.
• For second order reaction, $t_{1/2}=\dfrac{1}{k[A_0]},$ which depends on initial concentration of reactant.
44846.Among $CaH_2, BeH_2 , BaH_2,$ the order of ionic character is
$BeH_2 < BaH_2 < CaH_2$
$CaH_2 < BeH_2 < BaH_2$
$BeH_2 < CaH_2 < BaH_2$
$BaH_2 < BeH_2 < CaH_ 2$
Explanation:
For $2^{nd}$ group hydrides, on moving down the group metallic character of metals increases so ionic character of metal hydride increases. Hence the option (3) should be correct option.
For $2^{nd}$ group hydrides, on moving down the group metallic character of metals increases so ionic character of metal hydride increases. Hence the option (3) should be correct option.
44847.Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below :
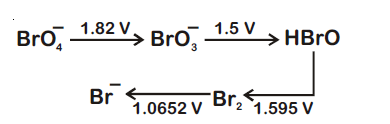
Then the species undergoing disproportionation is
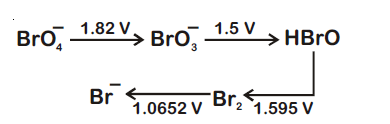
Then the species undergoing disproportionation is
$Br_2$
$BrO_4^-$
$BrO_3^-$
HBrO
Explanation:

= 1.595 – 1.5
= 0.095 V = + ve
Hence, option (3) is correct answer.
= 1.595 – 1.5
= 0.095 V = + ve
Hence, option (3) is correct answer.
44848.For the redox reaction
$MnO_4^-+C_2O_4^{2-}+H^+\rightarrow Mn^{2+} +CO_2 + H_2O$
The correct coefficients of the reactants for the balanced equation are
$MnO_4^-$ $C_2O_4^{2-}$ $H^+$
$MnO_4^-+C_2O_4^{2-}+H^+\rightarrow Mn^{2+} +CO_2 + H_2O$
The correct coefficients of the reactants for the balanced equation are
$MnO_4^-$ $C_2O_4^{2-}$ $H^+$
2 16 5
2 5 16
16 5 2
5 16 2
Explanation:

n-factor of $MnO_4^-\Rightarrow 5$
n-factor of $C_2O_4^{2-}\Rightarrow 2$
Ratio of n-factors of $4MnO_4^-$ and $C_2O_4^{2-}$ is 5 : 2. So, molar ratio in balanced reaction is 2 : 5.
∴ The balanced equation is
$2MnO_4^-+5C_2O_4^{2-}+16H^+\rightarrow 2Mn^{2+}+ 10 CO_2 +8 H_2O$
n-factor of $MnO_4^-\Rightarrow 5$
n-factor of $C_2O_4^{2-}\Rightarrow 2$
Ratio of n-factors of $4MnO_4^-$ and $C_2O_4^{2-}$ is 5 : 2. So, molar ratio in balanced reaction is 2 : 5.
∴ The balanced equation is
$2MnO_4^-+5C_2O_4^{2-}+16H^+\rightarrow 2Mn^{2+}+ 10 CO_2 +8 H_2O$
44849.Which one of the following conditions will favour maximum formation of the product in the reaction,
$A_2(g)+B_2(g)\rightleftharpoons X_2(g)\triangle_r H=-XKJ?$
$A_2(g)+B_2(g)\rightleftharpoons X_2(g)\triangle_r H=-XKJ?$
High temperature and high pressure
Low temperature and low pressure
Low temperature and high pressure
High temperature and low pressure
Explanation:
$A_2(g)+B_2(g)\rightleftharpoons X_2(g)\triangle H=-XKJ?$
On increasing pressure equilibrium shifts in a direction where pressure decreases i.e. forward direction. On decreasing temperature, equilibrium shifts in exothermic direction i.e., forward direction. So, high pressure and low temperature favours maximum formation of product.
$A_2(g)+B_2(g)\rightleftharpoons X_2(g)\triangle H=-XKJ?$
On increasing pressure equilibrium shifts in a direction where pressure decreases i.e. forward direction. On decreasing temperature, equilibrium shifts in exothermic direction i.e., forward direction. So, high pressure and low temperature favours maximum formation of product.
44850.When initial concentration of the reactant is doubled, the half-life period of a zero order reaction
Is tripled
Is doubled
Is halved
Remains unchanged
Explanation:
Half life of zero order
$t_{1/2}=\dfrac{[A_0]}{2K}$
$t_{1/2}$ will be doubled on doubling the initial concentration.
Half life of zero order
$t_{1/2}=\dfrac{[A_0]}{2K}$
$t_{1/2}$ will be doubled on doubling the initial concentration.
44851.The bond dissociation energies of $X_2 , Y_ 2$ and XY are in the ratio of 1 : 0.5 : 1. ΔH for the formation of XY is –200 kJ $mol^{–1}$. The bond dissociation energy of $X_2$ will be
$800 kJ mol^{–1}$
$100 kJ mol^{–1}$
$200 kJ mol^{–1}$
$400 kJ mol^{–1}$
Explanation:
The reaction for $Δ_fH^°(XY)$
$\dfrac{1}{2}X_2(g)+\dfrac{1}{2}Y_2(g)\rightarrow XY(g)$
Bond energies of $X_2, Y_2$ and XY are X, $\dfrac{X}{2}$, X respectively.
$\therefore \triangle H=\left(\dfrac{X}{2}+\dfrac{X}{4}\right)-X=-200$
On solving, we get
$\Rightarrow-\dfrac{X}{2}+\dfrac{X}{4}=-200$
$\Rightarrow X=800kJ/mole$
The reaction for $Δ_fH^°(XY)$
$\dfrac{1}{2}X_2(g)+\dfrac{1}{2}Y_2(g)\rightarrow XY(g)$
Bond energies of $X_2, Y_2$ and XY are X, $\dfrac{X}{2}$, X respectively.
$\therefore \triangle H=\left(\dfrac{X}{2}+\dfrac{X}{4}\right)-X=-200$
On solving, we get
$\Rightarrow-\dfrac{X}{2}+\dfrac{X}{4}=-200$
$\Rightarrow X=800kJ/mole$
44852.The correction factor ‘a’ to the ideal gas equation corresponds to
Electric field present between the gas molecules
Volume of the gas molecules
Density of the gas molecules
Forces of attraction between the gas molecules
Explanation:
In real gas equation, $\left(P+\dfrac{an^2}{V^2}\right)\left(V-nb\right)=nRT$ van der Waal’s constant, ‘a’ signifies intermolecular forces of attraction.
In real gas equation, $\left(P+\dfrac{an^2}{V^2}\right)\left(V-nb\right)=nRT$ van der Waal’s constant, ‘a’ signifies intermolecular forces of attraction.
44853.Consider the following species :
$CN^+, CN^–, NO$ and CN
Which one of these will have the highest bond order?
$CN^+, CN^–, NO$ and CN
Which one of these will have the highest bond order?
$CN^+$
$CN^–$
NO
CN
Explanation:
NO : $(\sigma 1s)^2, (\sigma^*1s)^2, (\sigma 2s)^2, (\sigma^*2s)^2, (\sigma 2p_z)^2, (\pi2p_x)^2$
$=(\pi 2p_y)^2, (\pi^*2p_x)^1=(\pi^*2p_y)^0$
$BO =\dfrac{10-5}{2}=2.5$
$CN^-:(\sigma 1s)^2, (\sigma^*1s)^2, (\sigma 2s)^2, (\sigma^*2s)^2, (\pi2p_x)^2$
$=(\pi2p_y)^2, (\sigma2p_z)^2$
$BO=\dfrac {10-4}{2}=3$
$CN:(\sigma 1s)^2, (\sigma^*1s)^2, (\sigma 2s)^2, (\sigma^*2s)^2, (\pi2p_x)^2$
$=(\pi2p_y)^2, (\sigma2p_z)^1$
$BO=\dfrac {9-4}{2}=2.5$
$CN^+:(\sigma 1s)^2, (\sigma^*1s)^2, (\sigma 2s)^2, (\sigma^*2s)^2, (\pi2p_x)^2$
$(\pi2p_y)^2$
$BO=\dfrac{8-4}{2}=2$
Hence, option(2) should be the right answer.
NO : $(\sigma 1s)^2, (\sigma^*1s)^2, (\sigma 2s)^2, (\sigma^*2s)^2, (\sigma 2p_z)^2, (\pi2p_x)^2$
$=(\pi 2p_y)^2, (\pi^*2p_x)^1=(\pi^*2p_y)^0$
$BO =\dfrac{10-5}{2}=2.5$
$CN^-:(\sigma 1s)^2, (\sigma^*1s)^2, (\sigma 2s)^2, (\sigma^*2s)^2, (\pi2p_x)^2$
$=(\pi2p_y)^2, (\sigma2p_z)^2$
$BO=\dfrac {10-4}{2}=3$
$CN:(\sigma 1s)^2, (\sigma^*1s)^2, (\sigma 2s)^2, (\sigma^*2s)^2, (\pi2p_x)^2$
$=(\pi2p_y)^2, (\sigma2p_z)^1$
$BO=\dfrac {9-4}{2}=2.5$
$CN^+:(\sigma 1s)^2, (\sigma^*1s)^2, (\sigma 2s)^2, (\sigma^*2s)^2, (\pi2p_x)^2$
$(\pi2p_y)^2$
$BO=\dfrac{8-4}{2}=2$
Hence, option(2) should be the right answer.
44854.Magnesium reacts with an element (X) to form an ionic compound. If the ground state electronic configuration of (X) is $1s^2 2s^2 2p^3$, the simplest formula for this compound is
$Mg_2 X$
$MgX_2$
$Mg_2X_3$
$Mg_3X_2$
Explanation:
Element (X) electronic configuration $1s^2 2s^2 2p^3$
So, valency of X will be 3.
Valency of Mg is 2.
Formula of compound formed by Mg and X will be Mg_3X_2.
Element (X) electronic configuration $1s^2 2s^2 2p^3$
So, valency of X will be 3.
Valency of Mg is 2.
Formula of compound formed by Mg and X will be Mg_3X_2.
44855.Iron exhibits bcc structure at room temperature. Above 900°C, it transforms to fcc structure. The ratio of density of iron at room temperature to that at 900°C (assuming molar mass and atomic radii of iron remains constant with temperature) is
$\dfrac{3\sqrt{3}}{4\sqrt{2}}$
$\dfrac{4\sqrt{3}}{3\sqrt{2}}$
$\dfrac{\sqrt{3}}{\sqrt{2}}$
$\dfrac{1}{2}$
Explanation:
For BCC lattice : Z = 2, $a=\dfrac{4r}{\sqrt 3}$
For FCC lattice : Z = 4, $a=2\sqrt{2}r$
∴$\dfrac{d_{25^°c}}{d_{900^°c}}=\dfrac{\left(\dfrac{ZM}{N_Aa^3}\right)_{BCC}}{\left(\dfrac{ZM}{N_Aa^3}\right)_{FCC}}$
$\dfrac{2}{4}\left(\dfrac{2\sqrt{2}r}{\dfrac{4r}{\sqrt{3}}}\right)^3$
$\left(\dfrac{3\sqrt{3}}{4\sqrt{2}}\right)$
For BCC lattice : Z = 2, $a=\dfrac{4r}{\sqrt 3}$
For FCC lattice : Z = 4, $a=2\sqrt{2}r$
∴$\dfrac{d_{25^°c}}{d_{900^°c}}=\dfrac{\left(\dfrac{ZM}{N_Aa^3}\right)_{BCC}}{\left(\dfrac{ZM}{N_Aa^3}\right)_{FCC}}$
$\dfrac{2}{4}\left(\dfrac{2\sqrt{2}r}{\dfrac{4r}{\sqrt{3}}}\right)^3$
$\left(\dfrac{3\sqrt{3}}{4\sqrt{2}}\right)$
44856.Which one is a wrong statement?
The electronic configuration of N atom is 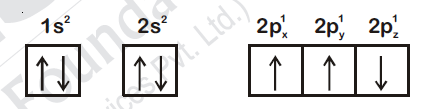

An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers
Total orbital angular momentum of electron in s orbital is equal to zero
The value of m for $d_z2$ is zero
Explanation:
According to Hunds Rule of maximum multiplicity, the correct electronic configuration of N-atom is

∴ Option (1) violates Hunds Rule.
According to Hunds Rule of maximum multiplicity, the correct electronic configuration of N-atom is
∴ Option (1) violates Hunds Rule.
